

Damage resulting from trauma or disease can result in debilitating sensory and motor deficits. Nerve injuries, particularly those resulting in significant gaps in the nerve tissue, pose a formidable challenge for clinicians and researchers. Despite their limitations, including limited availability and donor site morbidity, nerve autografts remain the clinical gold standard for treating nerve injuries.
Tissue engineering seeks to provide an alternative to the autograft with the fabrication of nerve repair constructs. These constructs have the potential to overcome the limitations of the autograft while still harnessing autograft biology with aligned biomaterials and therapeutic cells. A crucial aspect of nerve tissue engineering is the establishment of vascularization within the regenerating nerve tissue. This process plays a pivotal role in providing oxygen and nutrients to implanted cells, ensuring their long-term survival.
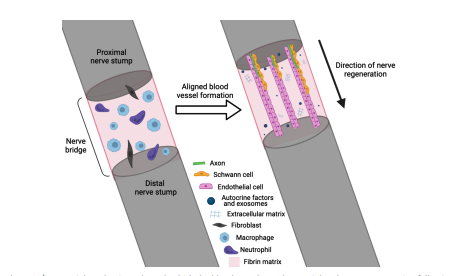
Over recent years, it has become ever more apparent that the role of blood vessels in nerve regeneration extends beyond vascularization. Blood vessels, and the endothelial cells that form the vessel inner lining, serve prominent structural, regulatory, and modulatory roles in nerve regeneration (Shen et al., 2004; Cattin et al., 2015; Grasman and Kaplan, 2017; Witjas et al., 2019; Fornasari et al., 2022; Huang et al., 2023). The exploitation of this knowledge has led to the development of various effective nerve injury treatments in animal models (Muangsanit et al., 2021; Thibodeau et al., 2022; Huang et al., 2023). Therefore, the intricate interplay between vascularization and nerve tissue engineering could be the key to improving engineered nerve repair constructs.
Vascularization is critical in regenerating many organs and tissues within the human body, including nerves, with blood vessel formation occurring throughout organ development and tissue repair. During angiogenesis, sprouting blood vessels form an organized structure within tissues serving as a transport network for oxygen, nutrients, metabolites, waste, and cells; all of which are essential for sustainable tissue regeneration.
Furthermore, endothelial cells actively regulate tissue homeostasis and repair. Endothelial cells secrete several growth factors necessary for tissue development and regeneration (Figure 1). Vascular growth factors released from endothelial cells contribute to the development of various tissues such as the heart, kidney, lung, bone, and nerve (Ramasamy et al., 2015). Shen et al. (2004) showed that secreted factors from brain endothelial cells activate Notch and Hes1 signaling, thereby promoting the selfrenewal of neural stem cells, implying the critical role of endothelial cells in the neural stem cell niche in the maintenance of the nervous system throughout life. Endothelial cell-secreted factors also benefit the peripheral nervous system. For example, human umbilical cord vein endothelial cells (HUVECs) release brain-derived neurotrophic factors that enhance the outgrowth of dorsal root ganglion neurons in vitro (Grasman and Kaplan, 2017).