

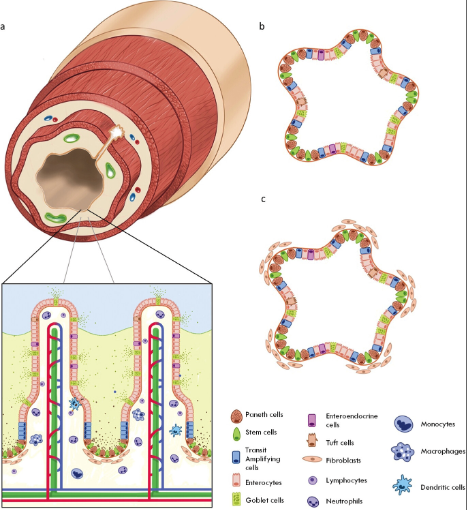
Together the intestine constitutes a selective barrier against the gut's contents and houses the largest portion of the microbiome in mammals. The intestine maintains this barrier by means of a single layer of secretory and absorptive intestinal epithelial cells (IECs), which are supported by an underlying stromal tissue containing matrix-secreting mesenchymal cells, neurons, smooth muscle, vasculature, and a plethora of immune cell types (Figure 1a).
(Figure 1 - The intestinal epithelium and intestinal organoids. a) Cross section of the small intestine with a zoomed schematic of the intestinal epithelium. b) Adult stem cell-derived intestinal organoid containing differentiated epithelial cells with both secretory and absorptive phenotypes. c) Embryonic or pluripotent stem cell-derived intestinal organoid containing differentiated epithelial cells and surrounding mesenchymal cells.)
Defective intestinal barrier function contributes to diverse pathologies including autoimmune diseases, neuropsychiatric disorders,[1] inflammatory bowel disease (IBD),[2, 3] cystic fibrosis,[4] and colorectal cancer (CRC).[5] Therefore, research models of the intestine are critical for understanding these diseases and informing drug development. However, animal models are limited by in vivo complexities and phylogenetic differences, and standard 2D cell culture models cannot re-create the complexity and diversity of the native tissue.
More than a decade ago it was shown that the intestinal epithelium (IE) could be reproduced in vitro as intestinal organoids (IOs) (Figure 1b,c). IOs can be cultured indefinitely as 3D structures that display native-like apicobasal polarity, and quickly proved to be accessible
tools to study intestinal development. Over time, IOs revealed insights into how genetic variants influence tissue homeostasis, and allowed for the mechanisms tissues harness to respond to damage to be uncovered. Importantly, IOs also offered new opportunities for researchers to model native tissue-like responses to disease. In short, IOs have overcome many drawbacks of traditional 2D cell culture and animal models, and have proven to be instrumental tools in intestinal research. However, most IOs consist of a single layer of epithelial cells, while the native IE is supported by numerous mesenchymal and immune cell types, which arrange themselves within a biochemically complex and structurally intricate extracellular matrix (ECM). Hence, researchers are developing new approaches that incorporate this plethora of physiological complexities into in vitro models of the intestine to answer questions that IOs alone cannot address.
Here, we discuss the cell types and matrix components that comprise the intestine and introduce research approaches to re-create them in vitro. We highlight strategies that rely on the inherent self-organization capacity of IOs for forming the epithelium, and present these in conjunction with designed approaches from bioengineering that aim to build tissues de novo. We discuss how biophysical cues within the native intestinal tissue, and the plethora of biochemical, gaseous, and microbial gradients in the intestine contribute to
normal function and can be replicated in vitro. We also highlight how new materials, including hydrogels with time-dependent and on-demand modulation of chemical and biological cues, can be used to mimic the physiochemical and mechanical properties of the native ECM. We end by providing a perspective on how further advances in biology might be combined with innovations in bioengineering to create advanced models of the intestine that can rival the accuracy of native tissues for applications in drug screening and personalized medicine.
The intestine consists of the epithelium that lines the lumen, as well as the underlying lamina propria and deeper submucosal and muscular layers, which together contain multiple mesenchymal cell types with distinct localizations and functions. This includes fibroblasts, myofibroblasts, pericytes, smooth muscle cells (SMCs), enteric neurons, endothelial cells, and immune cells. The intestine also contains a critical cell-free actor: the mucus. This glycoprotein network lines the luminal tube and acts as a first line of defence against mechanical, biological and chemical assaults.[6, 7] In addition, the gut houses trillions of microorganisms, which in conjunction with the intestinal mucosal immune compartment, tolerate and even use the microbiota to the advantage of the organ.
The intestinal epithelium maintains a strict barrier between the luminal contents and the rest of the body while still allowing for nutrient absorption. IECs achieve this by closely regulating the passage of luminal contents across their apical membrane, while simultaneously tightly sealing off intercellular spaces with selectively permeable tight junctions.[8] While the colonic epithelium is flat, the absorptive potential of the small intestine is maximized by luminal protrusions called villi. The inhospitable luminal environment demands continuous replacement of IECs. To achieve this, intestinal stem cells (ISCs) that reside within proliferative invaginations called crypts of Lieberkühn provide this source of cells.
The identity of ISCs remained controversial until lineage tracing revealed that the leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) was specifically expressed by cycling crypt-base columnar cells that can generate all epithelial lineages of the small and large intestine.[9] In the small intestine, ISCs reside at the base of crypts and fuel the turnover of the epithelium by continuous self-renewal or generation of daughter cells that rapidly proliferate before terminal differentiation. These daughter cells are either absorptive progenitors that differentiate into enterocytes, or are secretory progenitors that give rise to mucus-secreting goblet cells, antimicrobial peptide-secreting Paneth cells, hormone-secreting enteroendocrine cells, antigen-binding M cells and cytokine-secreting tuft cells.[10, 11] With the exception of Paneth cells, which migrate downwards intercalating between ISCs in the crypt, the other differentiated IECs migrate upward along the crypt-villus axis eventually reaching the villus tip, where they are shed into the lumen. This process feeds a cellular conveyor belt that replenishes the crypt-villus axis every 5–7 days in humans and maintains homeostatic barrier function.[8]
Stem cells within intestinal crypts replenish the epithelium; however, the ISC niche itself plays the more crucial role in this homeostatic process. The ISC niche comprises the physical and cellular microenvironment surrounding ISCs, which provides biochemical and mechanical cues that maintain ISC self-renewal, proliferation, and organized differentiation. Cells pushed out of the ISC niche are exposed to a signaling environment that promotes quiescence and differentiation, whereas cells that remain within the niche are proliferative and multipotent. Indeed, evidence suggests that the niche is so instructive that if space becomes available, even enterocyte precursors can revert into stem cells in response to niche signals.[12]