

This tone allows neuronal activity to evoke vasodilation that increases local cerebral blood flow (CBF). Much of the vascular resistance within the brain is located in capillaries and locus coeruleus axons have NA release sites closer to pericytes than to arterioles. In acute brain slices, NA contracted pericytes but did not raise the pericyte cytoplasmic Ca2+ concentration, while the α1 agonist phenylephrine did not evoke contraction.
Blocking α2 adrenergic receptors (α2Rs, which induce contraction by inhibiting cAMP production), greatly reduced the NA-evoked pericyte contraction, whereas stimulating α2Rs using xylazine (a sedative) or clonidine (an anti-hypertensive drug) evoked pericyte contraction. Noradrenaline-evoked pericyte contraction and capillary constriction are thus mediated via α2Rs. Consequently, α2Rs may not only modulate CBF in health and pathological conditions, but also contribute to CBF changes evoked by α2R ligands administered in research, veterinary and clinical settings.
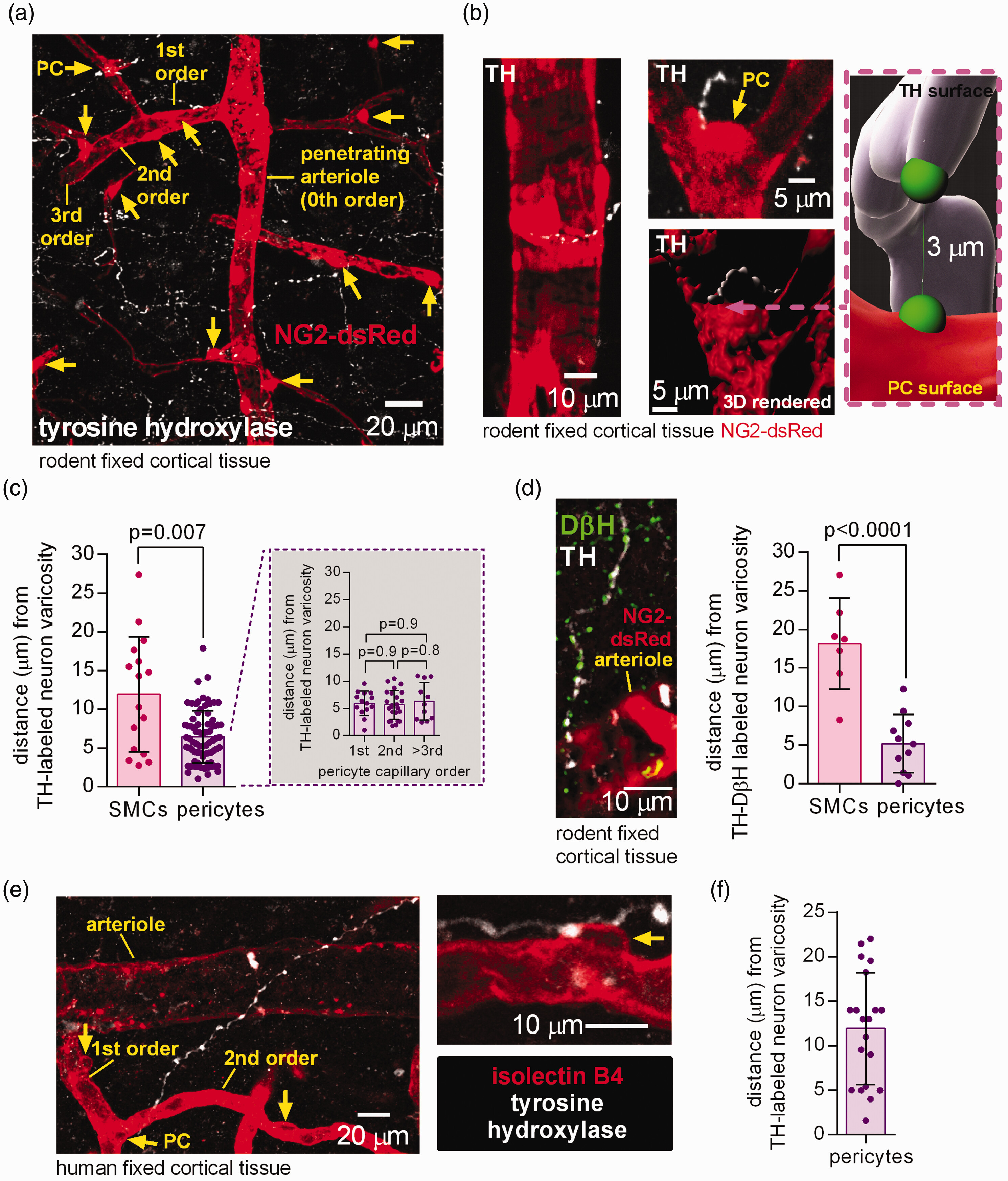
Active neurons require an increased energy supply to power their information processing. This is achieved by the neurons, and their associated astrocytes, releasing vasodilating agents that increase local cerebral blood flow (CBF).1 Within the cerebral cortex, although smooth muscle cells (SMCs) around arterioles contribute to regulating CBF, the majority of the vascular resistance is located in the capillary bed.2,3 Contractile pericytes, found especially on the first 3 branch orders of capillaries from arterioles (defined as 0th order), can adjust the capillary diameter to alter CBF, in health and in pathology.4–6
In order for CBF to be increased by neuronal activity, there must be an ongoing tone generated by contractile mural cells, which are then relaxed in order to increase vessel diameter. A significant contribution to this tone is generated by the release of noradrenaline from perivascular axon terminals of neurons with somata in the locus coeruleus and nearby nuclei.7,8 Two-thirds of these terminals are closer to capillaries rather than arterioles.9 Locus coeuruleus axons also target astrocytes,9 which have been suggested to be key intermediaries in the regulation of CBF by neuronal activity.4 Thus, locus coeuruleus derived noradrenaline may affect CBF by acting either directly on pericytes or SMCs, or indirectly via astrocytes. In brain slices, which presumably lack locus coeruleus noradrenaline release, superfusing noradrenaline constricts capillaries near pericyte somata (where the pericytes’ circumferential processes are mainly found6) by ∼60% in rat cerebellar cortex10 and by ∼40% in human cerebral cortex.6
Surprisingly, the mechanism by which noradrenaline contracts pericytes is unknown. An obvious possibility is that noradrenaline activates Gq-linked α1 receptors on pericytes which raise [Ca2+]i and thus activate contraction, as for arteriole smooth muscle.11 Alternatively, noradrenaline could act indirectly via astrocytes or neurons. For example noradrenaline raises astrocyte [Ca2+]i by activating α1 receptors,12 and these have been suggested to generate constriction of arteriolar SMCs by evoking the release of the arachidonic acid derivative 20-HETE (20-hydroxyeicosatetraenoic acid) from astrocytic endfeet enwrapping vessels.13
Here we use transgenic mice expressing NG2-dsRed in pericytes, and human tissue from neurosurgical operations, to investigate how noradrenaline evokes pericyte-mediated constriction of cerebral cortical capillaries. We confirmed that locus coeruleus axon noradrenaline release sites are located close to pericyte somata9 in both mice and humans. Surprisingly, however, we found that the constricting actions of noradrenaline were mediated, not by α1 receptors which raise [Ca2+]i, but by α2 receptors which lower the intracellular cyclic AMP concentration.