

Biofilms were first discovered centuries ago by Anton Von Leeuwenhoek, who used his microscope to examine tooth scrapings, but the first biofilm literature was not published until the 1970s, with the examination of “microbial films” taken from environmental water sources (Mack et al., 1975). However, the term “biofilm” was only introduced in the 1980s by Bill Costeron and collegues, which was used to describe microbial growth within medical samples (Lam et al., 1980; Marrie et al., 1982). More than 40 years on, we now realise that biofilms are the primary mode of growth for many bacterial and fungal species, which often form multikingdom communites with other organisms including protozoa and viruses (Burmølle et al., 2014). Therefore, understanding how biofilms form both inside and outide of the body has become an important aspect of modern medical research, such that new strategies can be developed to control environmental resevoirs of pathogens, and treat infectious diseases.
Biofilms go through a generalised life cycle. The classic model of biofilm development is a five-step process where biofilms resemble a mushroom-type shape. Planktonic cells (1) adhere, irreversibly, to surfaces which are primed with a conditioning film, followed by (2) proliferation of bacterial cells and an increase in biomass. This mass of cells then undergoes (3) maturation and (4) excretion of extracellular matrix. The final step is (5) dispersal of cells within the biofilm, allowing the dispersed planktonic cells the opportunity to adhere at other sites (Sauer et al., 2002; Stoodley et al., 2002). However, it is now thought that not all bacterial species grow in this classical way, especially within a native environment, and this concept has therefore been expanded on in recent years. A new conceptual model has been presented (Sauer et al., 2022), which instead suggests that there are 3 main stages of biofilm development; (1) aggregation and/or attachment, (2) growth and accumulation, and (3) disaggregation and/or detachment, with the possibility to aggregate and detach at any point and that these phases are affected by the environment and different conditions. This new model also accounts for non-surface-associated biofilms, particularly those observed in medicine, such as biofilms that form in the airways of patients with impaired mucociliary clearance (Sauer et al., 2022). Most importantly though, both models show that the maturation of biofilms, and the difference between simple aggregation and true biofilms, is defined by the excretion of extracellular polymeric substances (EPS), composed of carbohydrates, proteins, extracellular DNA (eDNA) and lipids (Di Martino, 2018).
Within the body a diverse microbial flora, arranged as biofilms, is associated with the skin and mucosal membranes, and these are highly beneficial to the host, for example within the gut (Macfarlane and Dillon, 2007), vagina (Leccese Terraf et al., 2016) and oral cavity (Robertson and McLean, 2015). However, dysbiosis can lead to changes within these biofilm communities, and can cause diseases such as inflammatory bowel disease (Baumgartner et al., 2021), bacterial vaginosis (Castro et al., 2019), and dental caries (Marsh, 2010), respectively. Bacteria can also cause a range of infections by forming biofilms on almost any indwelling medical device, including prosthetic joints, cardiac pacemakers, prosthetic heart valves, urinary catheters, and intravenous catheters (El Rafei et al., 2016; Beaver et al., 2021; Vilchez et al., 2021). These infections are often healthcare acquired (i.e. as a direct result of surgical contamination) but can also result from transient haematogenous spread of bacteria in the bloodstream (Honkanen et al., 2019). In addition, non-surface-associated bacterial aggregate biofilm infections are becoming more well-characterised (Alhede et al., 2011; Pabst et al., 2016; Cai, 2020), which are often chronic and low-grade, such as lung infections in cystic fibrosis patients (Høiby et al., 2010), chronic wound infections (Wei et al., 2019), and chronic otitis media (Akyıldız et al., 2013).
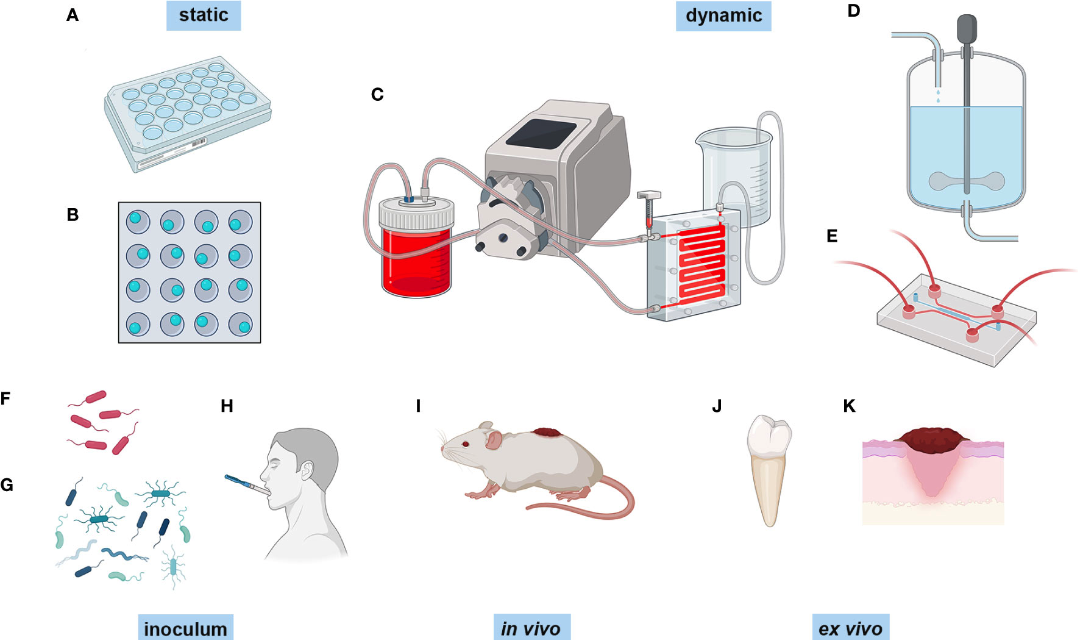
The type of infection being studied is important when choosing a biofilm model and screening technique, and some methods will be more appropriate than others. This review aims to discuss the different modelling techniques and the methods available for assessing functionality of biofilms.