

TRPV1 is activated by capsaicin (Tominaga et al., 1998), noxious heat (Caterina et al., 1997), noxious chemicals, including protons (Petersen and LaMotte, 1993) and peptide toxins (Siemens et al., 2006; Bohlen et al., 2010). TRPA1 in contrast is activated by noxious cold temperature (Story et al., 2003), mechanical stimuli (Kwan et al., 2006) and pungent compounds for example, allyl isothiocyanate (AITC) (Jordt et al., 2004).
TRPV1 and TRPA1 are expressed on small-diameter unmyelinated C-type and thinly myelinated Aδ-type fibres of the sensory ganglia, including dorsal root ganglia (DRG), trigeminal ganglia and nodose ganglia (NG) (Caterina et al., 1997; Story et al., 2003; Nagata et al., 2005). Within the gastrointestinal (GI) tract, TRPV1 and TRPA1 expression has been identified in the stomach, small intestine and colon of the rat, mouse and human, the major source originating from gut-projecting neurons of the NG and DRG (Tan et al., 2008; Holzer, 2011). Anterograde tracing of DRG to the mouse colon has shown spinal afferent terminals to be almost exclusively calcitonin gene-related peptide (CGRP) immunoreactive (Spencer et al., 2014) and these fibres are >90% immunoreactive to TRPV1 (Sharrad et al., 2015). The presence of TRPV1 in the enteric nervous system (ENS) however is controversial. Early studies suggest TRPV1 is present on intrinsic primary afferent neurons (IPANs), as well as on epithelial cells of the gastric and small intestinal mucosae (Anavi-Goffer et al., 2002; Kato et al., 2003; Faussone-Pellegrini et al., 2005; Holzer, 2011). However, numerous other studies have failed to identify TRPV1-immunoreactivity in enteric neurons (Patterson et al., 2003; Ward et al., 2003; Horie et al., 2004; Sharrad et al., 2015). On the other hand, TRPA1 has been identified on inhibitory motor neurons, descending interneurons, cholinergic neurons, and IPANs of the ENS (Poole et al., 2011) and on non-neuronal cells, for example, 5-hydroxytryptamine (5-HT)-secreting enterochromaffin (EC) cells and cholecystokinin-secreting enteroendocrine cells of the human and rodent GI mucosae (Purhonen et al., 2008; Nozawa et al., 2009).
In the GI tract, TRPV1 and TRPA1 modulate local functions such as mucosal anion secretion (Yarrow et al., 1991; Kaji et al., 2012; Fothergill et al., 2016), motor activity (Penuelas et al., 2007; Matsumoto et al., 2009; Nozawa et al., 2009) and vascular perfusion (Kono et al., 2013). Commonly, these functions are achieved via the release of neurotransmitters/peptides from afferent nerve endings which can act directly, stimulating different target cells e.g., epithelia and smooth muscle cells (de Man et al., 2008; Yarrow et al., 1991), or indirectly, through a neurogenic process, identified through atropine- and/or TTX-sensitivity, involving submucosal neurons (primarily involved in mucosal secretion) (Vanner and MacNaughton, 1995) and myenteric neurons (predominantly involved in GI motility) (Someya et al., 2003; Penuelas et al., 2007).
Of importance is the heterogeneity and regional differences in the receptors/channels expressed by neurons and EC cells (as well as the associated neuropeptide/transmitter contents) along the length of the GI tract (Martin et al., 2017; Drokhlyansky et al., 2020; Koo et al., 2021; Burclaff et al., 2022). For example, we have observed that mucosal responses to glucagon-like peptide 1 and CGRP showed significant intracolonic variability in mouse (Tough et al., 2018). Therefore, the signalling mechanisms following TRPV1 and TRPA1 activation are also likely to be region- (and species)-dependent. Furthermore, with functional evidence inferring both luminal and serosal TRPA1 activity it is also important to consider potential signalling sidedness in the mucosae. Most studies to date overlook these aspects, limiting our understanding of potential region- and/or side-dependence in TRP channel-mediated physiological functions along the colonic length, thus forming the main aims of this study.
AITC and (1E,3E)-1-(4-Fluorophenyl)-2-methyl-1-penten-3-one oxime (A967079) were gifted by Professor S. Bevan and Dr D. Andersson. Aprepitant and 6-Isopropoxy-9-xanthone-2-carboxylic acid (AH 6809) were purchased from Bio-Techne (Abingdon, UK), 1-[3,5-Dibromo-N-[[4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-1-piperidinyl]carbonyl]-D-tyrosyl-L-lysyl]-4-(4-pyridinyl)-piperazine (BIBN4096), capsazepine and tetrodotoxin (TTX) from Tocris (Bristol, UK), substance P and rat αCGRP from Bachem (St Helens, UK), 4-(4-,9-Diethoxy-1,3-dihydro-1-oxo-2H-benz[f]isoindol-2-yl)-N-(phenylsulfonyl) benzeneacetamide (GW627368) from Generon (Slough, UK) and prostaglandin E2 (PGE2) from Santa Cruz (Dallas, USA). Piroxicam, tropisetron, 1-[4-Amino-5-chloro-2-(3,5-dimethoxy-phenyl)methyloxy]-3-[1-[2-methylsulphonylamino]ethyl]piperidin-4-yl]propan-1-one hydrochloride (RS39604), 5-HT, 5-bromo-N-(4,5-dihydro-1H-imidazole-2-yl)-6-quinoxalinamine (UK 14,304) and capsaicin were all purchased from Sigma (Poole, UK). Forskolin were obtained from Abcam (Cambridge, UK). All small molecules were dissolved in neat dimethyl sulfoxide (DMSO, at 1 mM), capsaicin and capsazepine were dissolved in ethanol (EtOH; 95%) and peptides and toxin were dissolved in distilled water (dH2O). All stock aliquots were stored at -20°C until required and underwent one freeze-thaw cycle only, apart from AITC which was stored at +4°C.
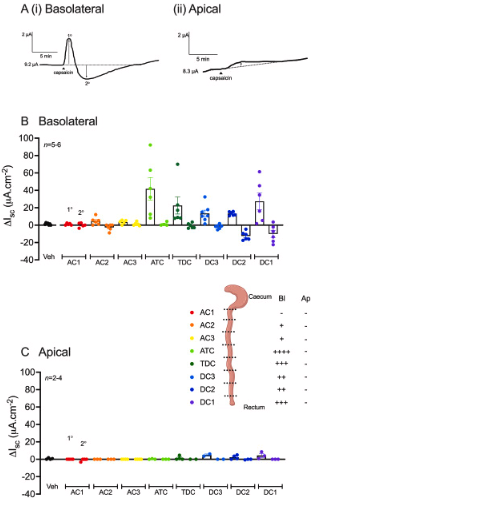
Mice (male and female C57BL/6J, 10+ weeks old, procured from Charles River Laboratories (Margate, UK)) had free access to standard chow (Rat and Mouse No.3 Breeding diet; Special Diets Services, Braintree, UK) and water ad libitum and were housed in 12 h light-dark cycles, with regulated temperature (22 ± 2°C) and humidity (55% ± 10%) settings. All animal care and experimentation was performed in compliance with the Animals (Scientific Procedures) Act 1986 and were approved by the UK Home Office (licence number: P6EA0199C). Mice were killed by cervical dislocation and the colon excised and submerged in fresh Krebs-Henseleit (KH; in mM; NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, and D-glucose 11.1). The colon was then cut along the mesenteric line and luminal contents removed. The tissue was pinned mucosal side down and the overlying smooth muscle and associated myenteric innervation was removed by blunt microdissection. The mucosa with intact submucosal innervation was segmented, generating 8 adjacent preparations designated as ascending colon (closest to the caecum; AC1-AC3), transverse colon (ATC and TDC) or descending colon (DC3-DC1; closest to the rectum) as described previously (Tough et al., 2018). Each preparation was positioned in an Ussing chamber (exposed area, 0.14 cm2) bathed on both sides in circulating aerated (95% O2/5% CO2) KH maintained at 37°C and voltage-clamped at 0 mV, as described previously (Tough et al., 2011). Once stabilisation of the basal short-circuit current (Isc; μA.cm-2) (20 min) and transepithelial resistance (TER, Ω.cm2; measured by delivering +1 mV pulses and applying Ohm’s law) was achieved, agonist or antagonist additions were made to the apical (ap) or basolateral (bl) reservoirs as specified.
To investigate response sidedness, the TRPV1 agonist, capsaicin (1 μM) or the TRPA1 agonist, AITC (10 μM) was applied bl or ap to 8 adjacent naïve colonic preparations (AC1-DC1 as described in section 2.2) and the changes in Isc recorded over 40 min.
Selective antagonism studies utilised capsazepine (TRPV1 antagonist) or A967079 (TRPA1 antagonist). Capsazepine (3, 10, 30, 100 μM) or vehicle (0.1% EtOH) were applied bl (only) to 5 adjacent colonic preparations (taken within the TDC-DC1 region) 20 min prior to capsaicin (1 μM; bl) and the maximum changes in Isc were recorded. Alternatively, A967079 (1, 3 or 10 μM) or vehicle (0.1% DMSO) were applied bl to the ascending colon (4 adjacent preparations taken within the AC1-AC3 region) or ap to the descending colon (4 preparations taken within DC3-DC1) 20 min prior to a single bl (ascending colon) or ap (descending colonic region) AITC (10 μM) addition and the changes in Isc recorded for 30 min.
To investigate TTX-sensitivity, the neurotoxin (100 nM; bl) or vehicle (dH2O) were applied to 4 adjacent colonic preparations taken from the TDC-DC1 region, or 4 adjacent preparations taken within the AC1-AC3 region 20 min prior to a capsaicin (1 μM; bl, descending region only) or AITC (10 μM; ap) addition, and the changes in Isc recorded.